Multiple epigenetic markers have been determined as determinants for prognostic values in prostate cancer, PCa. There are two recent papers, one of PCA3 and its pathway control, and on EZH2 and its use as a marker. We briefly summarize these efforts and attempt to place them in a common and ever growing context of both prognostic markers as well as putative pathway control therapeutic targets.
PCA3
PCA3 has received a great deal of attention of late. It is a
non-coding RNA and the controlling gene is located at 9q21-q22[1].
It is also called prostate cancer antigen 3 (non-protein coding). The presence
of PCA3 is generally now believed to be a marker for PCa. Testing is now
underway on may patients to determine if they have PCa using the PCA3 assay. Thus
there is a great deal of interest in better understanding what the full
networks are for PCA3 generation as well as looking at those pathways as a
possible means to control PCa. We examine two recent studies in this area.
In the recent paper by Ferreira et al, they state:
Our findings suggest that the ncRNA PCA3 is
involved in the control of PCa cell survival, in
part through modulating AR signaling, which
may raise new possibilities of using PCA3
knockdown as an
additional therapeutic strategy for PCa control.
This may be of
significant merit as a new potentially useful therapeutic. Now it should be
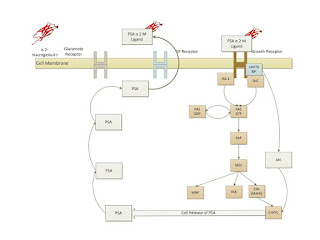
recalled that the AR pathway and the PSA generation is known as shown below[2].
Now Ferreira et al continue:
Due to the increased PCA3 expression in
androgen-responsive cells compared with androgen-insensitive cells, and because
AR signaling is an important pathway controlling PCa survival, we tested
whether PCA3 expression was modulated by the androgen-active metabolite DHT and
whether this expression pattern involved the activated AR.
Upregulation of PCA3 expression in response to LNCaP
stimulation with DHT was significantly counteracted by the AR antagonist
flutamide, indicating that PCA3 expression was induced by the activated AR. AR
activation was further confirmed by the observation that LNCaP cells stimulated
with DHT also showed AR transcriptional activity. Consistently, all of the AR
target genes tested that contain canonical AR response elements (AREs) in their
promoter sequences, were upregulated upon DHT treatment. Although eight of the
genes showed at least a 1.5-fold increase after AR activation, only two of them
showed a significant increase in their expression levels. Interestingly, PCA3
upregulation upon DHT treatment has been observed previously, but no study has
demonstrated the involvement of activated AR in PCA3 expression by using AR
antagonists. Although our data also suggest that PCA3 is an androgen-responsive
gene, the precise molecular mechanism by which PCA3 expression responds to this
activation is still unknown.
One hypothesis is that activated AR can directly activate
the PCA3 promoter, as has been demonstrated for the miR-101 and miR- 21
regulatory regions, which are also modulated by the activated AR. However, no
consensus AREs have been identified in the 500-bp PCA3 promoter region. We
further screened for consensus ARE elements in the entire PCA3 genomic region
at the 5 Kb region upstream from the PCA3 transcription start site, and have so
far identified no canonical element (data not shown). Nevertheless, we cannot
exclude the possibility that other, noncanonical ARE elements could also
promote AR binding and directly activate PCA3 expression, as has been
previously described for other genes modulated by the AR activation. PCA3-upregulated
expression in response to DHT treatment could also be a result of activated AR
binding to the regulatory regions of other AR-responsive genes, which in turn
could induce PCA3 expression. Further experiments should investigate direct AR
binding to different PCA3 genomic regions, in order to answer these open
questions.
Now they examined genes which are known pathway controllers
of PCa. The CDKs especially control cell cycle flow.
As an approach to investigate the signal by which PCA3
controls PCa cell survival, we analyzed the transcript expression of PSA, AR,
TMPRSS2, NDRG1, GREB1, FGF8, CDK1, CDK2, and PMEPA1 genes, all of which have
key roles in PCa growth and progression, and are classical AR target genes.
Also highly regulated by androgens, fibroblast growth
factor 8 (FGF8), cyclin-dependent kinase 1 (CDK1), cyclin-dependent kinase 2
(CDK2), and the gene regulated in breast cancer 1 (GREB1) gene products have
classical stimulating roles in prostate growth and proliferation. Conversely,
the PMEPA1 gene, although a direct transcriptional target of the AR, has been
described as a negative regulator of cell growth in the prostate epithelium, as
well as negatively regulating AR protein levels in different cell-culture
models. We also observed that the AR transcription level was downregulated
after PCA3 knockdown. These results accord with previously published data,
which demonstrated that the AR gene is transcriptionally regulated by AR
through binding to AR regulatory elements (autoregulation). However,
differently from the other AR-responsive genes tested here, the ARE elements
required for this process have not been found in the AR promoter or in the
5'-flanking region, but rather in AR coding sequences.
The observation that PCA3 is involved in the control by
modulation of the AR target genes is a key observation. As we have shown, based
upon various prior works, the change in AR is critical to the loss of any
control over the PCa cells. They state:
Here we demonstrate for the first time that PCA3 is
involved in the control of PCa cell survival, at least in part by modulating
the transcriptional activity of AR target genes. To our knowledge, this is the
first characterization of the functional role of PCA3 in PCa cells, and will
not only improve the understanding of key roles of this transcript in prostate
carcinogenesis, but also suggests an alternative strategy to use PCA3 as a
putative specific target for PCa treatment approaches. Because PCA3 seems to be
a regulator of the expression of AR target genes and PCa cell survival,
treatment options aiming to downregulate PCA3, in combination with other
androgen-depletion-based strategies, could potentially circumvent
androgen-ablation resistance mechanisms.
In an earlier paper by Ferreira et al, they state:
The prostate cancer antigen 3 (DD3/PCA3) is a non-coding
RNA (ncRNA) specifically expressed in prostate tissues and overexpressed in
prostate cancer (PCa) tumors. Although widely applied as a diagnostic marker
for PCa, to date nothing has described about its role in PCa biology. We used
herein small interfering RNA (siRNA) in order to knockdown DD3 mRNA message as
an approach to elucidate DD3 functional roles in PCa cells.
LNCaP cell line was been used herein as an in vitro model
for DD3 functional assays. siRNA sequences were specifically designed for DD3
exon 4 mRNA sequences (siDD3), as well an scrambled siRNA (siScr), as negative
control. LNCaP cells were transiently transfected with siDD3 or siScr and DD3
expression was analysed by real time PCR (qRT-PCR) using DD3 specific
oligonucleotides. LNCaP cells transfected with siDD3 demonstrated a marked
decrease in cell proliferation and viability, as compared to siScr transfected
cells.
Further, LNCaP cells in which DD3 was knocked-down
presented a significant increase in proportion of cells in SubG0/G1 phase of
cell cycle and presenting pyknotic nuclei, indicative of cells undergoing
apoptosis. In order to investigate the putative mechanisms underlying the
decrease of LNCaP cell survival as a result of DD3 knockdown, we then evaluated
the involvement of DD3 on androgen receptor (AR) pro-survival signaling. DD3
expression was significantly uregulated as a result of LNCaP treatment with
dihydrotestosterone (DHT), the active androgen metabolite. This effect was
reverted by the addition of the AR antagonist, flutamide.
Consistent to an AR activation by DHT treatment, LNCaP
cells presented a significant upregulation of AR target genes. Notably,
siDD3/LNCaP transfected cells significantly inhibited the expression of tested
AR responsive genes. Besides, DD3 knockdown was able to counteract DHT
stimulatory effects over AR target gene expression. Despite negatively
modulating the transcription of AR target genes, DD3 knockdown did not alter
Akt and ERK phosphorylation, suggesting that DD3 is mainly controlling the
expression of signaling pathways downstream to AR activation.
In summary, our findings indicate that DD3 is a ncRNA
whose expression is AR regulated and is involved on the control of PCa cell
survival and proliferation, in part by modulating the AR signaling pathway and
its target genes.
These findings correspond to the first description of DD3
roles on PCa cells and could provide new insights into understanding prostate
carcinogenesis, besides opening new prospects to use DD3 not only as a
biomarker for PCa, but also as an specific target for therapeutic approaches
aiming to inhibit PCa growth by negatively modulating AR pro-survival signal
and their target genes.
In this slightly earlier paper the authors focus on the PCA3
as a target and examine its pathway significance.
Other researchers have examined PCA3 as well as other
markers. It is well known that the TMPRSS2:ERG fusion is often seen in PCA. As Salagierski
and Schalken conclude:
In recent years advances in genetics and biotechnology
have stimulated the development of noninvasive tests to detect prostate cancer.
Serum and urine molecular biomarkers have been identified, of which PCA3 has
already been introduced clinically.
The identification of prostate cancer specific genomic
aberrations, ie TMPRSS2:ERG gene fusion, might improve diagnosis and affect
prostate cancer treatment. Although several recently developed markers are
promising, often showing increased specificity for prostate cancer detection
compared to that of prostate specific antigen, their clinical application is
limited. The only 2 true prostate cancer specific biomarkers identified to date
remain PCA3 and TMPRSS2:ERG gene fusion.
Let us briefly summarize these two genes and their fusion.
TMPRSS2:ERG
The TMPRSS2-ERG fusion is the single most seen molecular
lesion in prostate cancer. (see Taylor et al 2010) TMPRSS2 is on 21q22.3 and
ERG is on 21q22.3. Both are dominant. Unlike the pathway disturbances, this is
a fusion, translocation on the same gene, and the resultant is expressive of ERG and not of TMPRSS2.
Transcriptional regulator ERG is a protein that in
humans is encoded by the ERG gene (Ets Related Gene,
Chromosome 21). ERG is a member of the
ETS family of transcription factors.
Transcriptional regulator ERG is a nuclear protein that
binds purine-rich sequences. ERG can
fuse with TMPRSS2 protein to form an oncogenic fusion gene that is commonly
found in human prostate cancer, especially in hormone-refractory prostate
cancer. This suggests that ERG overexpression
may contribute to development of androgen-independence in prostate cancer
through disruption of androgen receptor signaling.
Transmembrane protease, serine 2 is an enzyme that in
humans is encoded by the TMPRSS2 gene. This gene encodes a protein that
belongs to the serine protease family. The encoded protein contains a type II
transmembrane domain, a receptor class A domain, a scavenger receptor
cysteine-rich domain and a protease domain. Serine proteases are known to be
involved in many physiological and pathological processes. This gene was demonstrated to be up-regulated
by androgenic hormones in prostate cancer cells and down-regulated in
androgen-independent prostate cancer tissue. The protease domain of this
protein is thought to be cleaved and secreted into cell media after
autocleavage. The biological function of this gene is unknown. TMPRSS2
protein's function in prostate carcinogenesis relies on overexpression of ETS
transcription factors, such as ERG and ETV1 through gene fusion. TMPRSS2-ERG
fusion gene is the most frequent, present in 40% - 80% of prostate cancers in
humans.
As Weinberg notes:
In the case of the TMPRSS-ERG fusion, both genes are
located on 21q22, and the fusion frequently occurs because of an interstitial
deletion . The resultant fusion transcripts are androgen responsive and usually
encode an ETS gene (ERG) truncated at its N terminus without any coding
elements from TMPRSS2. It is unknown if the biologic consequences of
misexpression of the truncated ETS family protein are different from expression
of the full length protein and whether truncation contributes to oncogenicity.
(Ref Weinberg)
The PCA3 gene is highly overexpressed in specific PCa
cell lines and prostatic tumours. In 2006, a simple and robust urine test
(Progensa) became commercially available. Despite its costs, prostate cancer
antigen 3 (PCA3) is superior to prostate-specific antigen (PSA) and percent
free PSA in the early detection of PCa. PCA3 improves the diagnostic accuracy
of externally validated nomograms among men with an elevated PSA undergoing
biopsy.
PCA3 independently predicts low-volume disease and
pathologically insignificant PCa but is not associated with locally advanced
disease and is limited in the prediction of aggressive cancer. Preliminary data
demonstrate that combining PCA3 with other new biomarkers further improves
diagnostic and prognostic accuracy.
Finally, findings of the first PCA3-Gene-ViroTherapy
study suggest therapeutic potential by exploiting PCA3 overexpression. PCA3,
integrated in novel biopsy nomograms or risk stratification tools, can be used
to counsel or confirm biopsy indications. If confirmed in further studies,
using PCA3 together with established staging risk factors could assist
clinicians in specific pretreatment decision making. So far no evidence for the
usefulness of PCA3 in active surveillance programs has been presented.
The above seems to indicate that although PCA3 is indicative
of PCa in low volume states but they state that it is not a metric for high
volume states. Other work appears to provide added light of PCA3 and may change
this observation.
We look at a recent thesis presented by Lee specifically on
a more detailed analysis of PCA3. From Lee we have:
Proposed mechanism of action of PC-TSGC toward the downregulation
of signal transduction of Rho GTPase family members. PC-TSGC inhibits the
binding of RhoA to its activator Lbc-RhoGEF by direct interaction with RhoA
through the BCH domain, and recruits nm23- H1 which in turn inhibits Tiam1, a
specific Rac activator. GEF: Guanine nucleotide Exchange Factor; GAP: GTPase
Activating Protein.
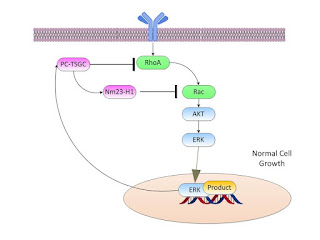
From Lee p 89 we have:
Proposed biological roles of PCA3 and PC-TSGC in prostate
cancer.
(A) Normal cell: growth stimuli are signaled to the
nucleus through multiple pathways that include activation of RhoA and Rac and
subsequent phosphorylation of AKT and ERK1/2. The signal transduction cascade
stimulates gene expression in order to initiate cellular replication and
inhibit apoptosis. Simultaneously, the same signals elicit the expression of
PC-TSGC which in turn inhibits RhoA and Rac (through nm23-H1), thereby
resulting in a negative-feedback loop on the activity of cell growth signaling
pathways.
(B) Cancer cell: in a malignant cell, the same mechanism
is altered by the abnormal expression of PCA3, which opposes the expression of
PC-TSGC. As a result, the control over the RhoA and Rac signaling pathways is
lost, and the cell engages an unregulated cell growth that potentially leads to
oncogenic transformation.
Now for abnormal cell growth we have:
We now examine another recent marker which also acts in an
epigenetic manner, specifically EZH2. EZH2 (located at 7q35-q36) is a member of
the Polycomb group, members of which are often associated with the silencing of
genes. The epigenetic capabilities allow it to block the expression of multiple
genes which are useful in normal cell homeostasis.
As NCBI states[3]:
This gene encodes a member of the Polycomb-group (PcG)
family. PcG family members form multimeric protein complexes, which are
involved in maintaining the transcriptional repressive state of genes over
successive cell generations. This protein associates with the embryonic
ectoderm development protein, the VAV1 oncoprotein, and the X-linked nuclear
protein. This protein may play a role in the hematopoietic and central nervous
systems. Multiple alternatively splcied transcript variants encoding distinct
isoforms have been identified for this gene.
PCa can move from Androgen responsive to Androgen resistant
by the blocking of certain genetic controls and also the activation of others. Simply
we see the three step process as follows:
First the normal cell operation is as shown below:
Then when the cell becomes cancerous, we see the expression
of the cancerous genes but they are supported by activated AR products. Often
in this stage we still have a localized stage and by depriving the androgen the
AR are suppressed in their activation.
Finally we can get to the androgen resistant state as we
show below. Several things happen here. First, androgen is actually produced to
self-sustain the malignant cell. Second, mutant AR cells can activate
independent of the presence of androgens. Third AR proteins can become enhanced
with specific sensitivity. The cell then
becomes resistant to any reduction of cell exogenous androgen availability.
This stage of PCa then becomes the most aggressive.
As is reported in Science, EZH2 has been seen to have
special significance in AR resistant PCa. They state:
Epigenetic regulators are implicated in cancer
progression and proposed as therapeutic targets. Xu et al.
report that EZH2 (Enhancer of zeste homolog 2), a factor previously
thought to exert its oncogenic function primarily as part of the polycomb repressive
complex, acts through a distinct mechanism in cells of castration-resistant
prostate cancer. Rather than exclusively silencing gene expression through
histone methylation, EZH2 acts as a transcriptional coactivator. The activation
function of EZH2 plays a critical role in the growth of castration-resistant
prostate cancer cells, which could be relevant in future drug development.
Xu and the authors state:
Epigenetic regulators represent a promising new class of
therapeutic targets for cancer. Enhancer of zeste homolog 2 (EZH2), a subunit
of Polycomb repressive complex 2 (PRC2), silences gene expression via its
histone methyltransferase activity. We found that the oncogenic function of EZH2
in cells of castration-resistant prostate cancer is independent of its role as
a transcriptional repressor. Instead, it involves the ability of EZH2 to act as
a coactivator for critical transcription factors including the androgen
receptor. This functional switch is dependent on phosphorylation of EZH2 and
requires an intact methyltransferase domain. Hence, targeting the non-PRC2 function
of EZH2 may have therapeutic efficacy for treating metastatic,
hormone-refractory prostate cancer.
Again a targeting of the AR resistant form of PCa has a
potential target in this protein. They conclude:
This study demonstrates that phosphorylation of EZH2 at
Ser21, mediated directly or
indirectly by the PI3K-Akt pathway, can switch its function from a Polycomb
repressor to a transcriptional coactivator of AR (and potentially other
factors). Rescue experiments and the lack of correlation with H3K27me3 levels
support a role for EZH2-directed methylation of substrates other than H3K27,
including potential nonhistone proteins. The current rationale for EZH2
inhibitor design is based primarily on targeting its Polycomb-repressive
activity and uses H3K27me3 as the pharmacodynamic readout.
However, the observed loss-of-function mutations of EZH2
inmyelodysplastic syndrome and acute leukemia raise concerns that such
inhibitors might exhibit important hematologic side effects.
Our finding of an altered function for EZH2 in CRPC cells
raises the potential to develop inhibitors that specifically target the EZH2
activation function while sparing its PRC2-repressive function. In addition,
our finding that EZH2 cooperates with AR-associated complexes and requires
phosphorylation to support CRPC growth suggests novel combination therapies for
the treatment of metastatic, hormonerefractory prostate cancer.
Thus they contend that developing a therapeutic for this
specific product could address the AR instabilities.
References
1.
Auprich M, et al,
Contemporary role of prostate cancer antigen 3 in the management of prostate
cancer, Eur Urol. 2011 Nov;60(5):1045-54. doi: 10.1016/j.eururo.2011.08.003.
Epub 2011 Aug 25.
2.
Ferreira, L. et al,
DD3/PCA3 non-coding RNA regulates prostate cancer cell survival and modulates
AR signaling, Cancer Research: April 15, 2012; Volume 72, Issue 8,
Supplement 1 , Proceedings of the 103rd Annual Meeting of the American
Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, IL. Philadelphia
(PA): AACR; Cancer Res 2012;72(8 Suppl):Abstract nr 201., http://cancerres.aacrjournals.org/cgi/content/short/72/8_MeetingAbstracts/201?rss=1
.
3.
Ferreira, L. et al, PCA3 noncoding RNA is involved in the
control of prostate-cancer cell survival and modulates androgen receptor
signaling, BMC Cancer 2012, 12:507; http://www.biomedcentral.com/content/pdf/1471-2407-12-507.pdf
4.
Lee, A., A NEW TUMOR
SUPPRESSOR GENE CANDIDATE REGULATED BY THE NONCODING RNA PCA3 IN HUMAN PROSTATE
CANCER, (2010). Univ Texas GSBS Dissertations and Theses, PhD, (Open Access). http://digitalcommons.library.tmc.edu/cgi/viewcontent.cgi?article=1047&context=utgsbs_dissertations
5.
Salagierski M, Schalken JA.,
Molecular diagnosis of prostate cancer: PCA3 and TMPRSS2:ERG gene fusion. , J
Urol. 2012 Mar;187(3):795-801. doi: 10.1016/j.juro.2011.10.133. Epub 2012 Jan
15.
6.
Weinberg, R., Cancer,
Garland (New York) 2008.
7.
Xu, K. et al., EZH2 Oncogenic Activity in
Castration-Resistant Prostate Cancer Cells Is Polycomb-Independent, Science 338,
1465 (2012).
[2]
Note we use the reference, Prostate Cancer Genomics, McGarty (2012, DRAFT, http://www.telmarc.com/Documents/Books/Prostate%20Cancer%20Systems%20Approach%2003.pdf
) as the source for some of this information. From this source one may obtain
the initial sources.