Rationalism and Empiricism may be two ends of the same
process. Empiricism is “knowing” by observing facts, and that alone leads to
knowledge. Rationalism assumes inherently that the human intellect can through
logic attain new knowledge. Galen in his writings and his approached to
medicine espoused the amalgam of both the empirical and rational. Empirically
there are observations of facts. Rationally we can then relate those facts in a
logical construct and within that construct we can attempt to ascertain new
understanding. Oftentimes the "facts" is an observation lacking in
the interconnecting "facts" but through a logical construct and
subsequent validation we can then construct a valid sequence that demonstrates
how best to attract a disorder[1].
In a recent examination of PCa there is an interesting
blending of both the rational and empirical. We use the brief discussion of
prostate neuroendocrine functioning from the paper in NEJM by Chen and Ayala
who note:
Thirty years ago, Sir
James W. Black shared the Nobel Prize in Physiology or Medicine for his
contribution to the development of propranolol (a beta-blocker) and cimetidine
(a histamine H2 blocker). Since that time, beta-blockers have been and remain
widely used as antihypertensive drugs. An interesting side effect of these drugs
is a reduction in the risk of prostate cancer and associated death. Thus, there
exists an epidemiologic link between a drug that affects the adrenergic nervous
system and prostate tumorigenesis.
This statement provides
an interesting example of examining the above mentioned interplay of
rationalism and empiricism in cancer diagnosis and treatment. Namely we have
the empirical relationship between beta blockers, a therapeutic that works on
the neurological system's control of other cells, and the unregulated cell
growth of prostate cancer.
Namely we look at
neuroendocrine type effects and thus it requires a slightly more detailed
understanding of the prostate As NCI notes[2]:
Neuroendocrine: Having
to do with the interactions between the nervous system and the endocrine
system. Neuroendocrine describes certain cells that release hormones into the
blood in response to stimulation of the nervous system.
We then, in a rationalistic manner, can try and connect the
other empirical facts and see if the initial observation can also be logically
correct and from that logic ascertain a new therapeutic approach.
A simplistic view of a neuroendocrine system is shown below.
Basically the neuro cell activates the endocrine cell which in turn sends out
signals to other collections of cells to do whatever they are supposed to do.
The above is simplistic but based upon a substantial base of
validated cellular signalling factors. Namely these results are empirical in a
broad sense. Now when examining various cancers we often look at the cancer
cell as being the driving factor. However in a neuroendocrine environment, the
cancer cell may be getting its signalling from a cancer initiating cell which
in turn is being signaled by a neuro cell. The cancer initiating cell may be
blocked by blocking the signalling between it and the causative neuro cell.
That is the logical or rationalistic part of this exercise.
The questions now are;
(i) If the malignancy occurs in the neuroendocrine cell,
then does it create an environment for proliferation of other cells?
(ii) If the malignancy occurs in the neuroendocrine cell
does it send out signals that either block other homeostatic processes or does
it accelerate angiogenesis in the new malignancy?
(iii) If the malignancy starts in a non-neuroendocrine cell,
are there processes that effectively "turn on" the neuroendocrine
cell to facilitate such effects as proliferation, angiogenesis, gene
suppression or activation in other cells?
These are but a few of the questions which may be posed.
Again we indicate that this is a bit simplistic but it does present the key
issues related hereto.
The process of blending rationalism and empiricism in this
specific case is accomplished as follows:
1. A set of basic facts are assembled.
2. The basic facts are assembled in some logical manner.
3. Missing links are identified
4. New facts are obtained
5. The logical process is reiterated
6. This proceeds until a conclusive result is obtained.
Let us summarize some of the Basic Facts:
1.
PCa is common among men
being the most significant cancer in older males.
2.
The prostate is a highly
enervated organ.
3.
The prostate is
fundamentally a glandular organ having many small glandular structures with
basal cells and luminal cells.
4.
However the prostate also
contain a small percentage of cells activated by nerve cells via such ligands
as those activated by nerve cell activating molecules.
5.
The activation of these
neuroendocrine cells, the prostate cells activated by neurons, then results in
a variety of actions in other cells by means of an endocrine like action.
6.
PCa is seen as a
progressive malignancy starting in the proliferation of the basal and luminal
cells and the proliferation
7.
The most aggressive PCa is
neuroendocrine PCa.
8.
The neuroendocrine actions
overcome androgen control leading to CRCP, castration resistant prostate
cancer.
9.
If one can disable the
neuroendocrine activity then perhaps PCa can be controlled.
10. Beta blockers control neuroendocrine activity.
11. Thus beta blockers may be effective against PCa.
This supposition we explore in some detail herein.
We first examine neuroendocrine cells. Fundamentally as
discussed above they are cells which interact with the nerves and in turn have
an endocrine type function releasing molecules whose effect results in change
to other cells.
From Li et al we have:
Neuroendocrine ("NE")
cells are found in many tissues including normal prostate. NE cells in normal
prostate, though a small subset of cells, are randomly interspersed amongst the
luminal and basal cells of the prostate glands in all anatomic zones, with a
slight more cells in transitional zone and peripheral zone than that in central
zone. They are not readily
recognized under the light microscope using conventional hematoxylin and eosin
staining, but can be easily demonstrated by immunohistochemical staining with
specific markers, such as Syn, CgA and CD56 etc. Under electron microscope,
there are two different morphologic types of NE cells: the open-type cells and
the closed-type cells. The open-type cells
possess long surface microvilli through which the cells reach the lumen and
receive luminal stimuli (pH, chemicals). The closed-type cells have lateral
processes like dendritic cells through which the cells can contact the adjacent
epithelial cells (luminal cells and basal cells), and receive stimuli from
nerve endings, neighboring blood vessels and underlying stromal cells. The different
morphologic types of NE cells are found to distribute differently in the
prostate and seminal vesicles when the topography and structure of the
excretory ducts of the different glands are analyzed in male rats. Approximately 40% of
the NE cells of the ventral prostate ducts are of the open-type, whereas 14% of
the seminal vesicle ducts, where most of the NE cells are of the closed-type.
The finding suggests that the distribution pattern and different morphologic
types of NE cells may be associated with different function
We can obtain a simplistic understanding as follows. The
prostate is filled with glandular structures as shown below composed of basal
cells at the base (blue cells) and luminal cells (red cells) looking inward to
the gland.
However the prostate is filled with many nerves and certain
of these cells are the neuroendocrine cells, namely part of the gland but
controllable by the nerve cells surrounding them. We simplistically depict this
below[3]. We show the gland as
previously described but the neuroendocrine cell is in orange and the neuron in
light blue.
Note above the neuroendocrine cell may participate in the
normal structure of the prostate but that it communicates via neurotransmitters
with the nerves. These cells are part of the process of sending prostatic fluid
out with semen and other such fluids. Identifying these cells is complex
because of the need to use certain staining methods and these cells were only
identified in the last few decades.
Now the entire prostate may look as follows where there are
many glandular cells and many additional nerve fibers. One must remember that
the prostate is highly innervated.
There are many nerves and many small glandular structures
and the neuroendocrine cells participate in the overall innervation process.
As Feldman and Feldman have noted:
The main function of the prostate is to produce seminal
fluid. The prostate is made up of epithelial glands and a fibromuscular stroma.
The glandular epithelium, which gives rise to prostate adenocarcinoma, has
three types of cells: basal, luminal secretory and neuroendocrine. There are fewer basal cells and their function is not
fully understood, although they secrete components of the basement membrane. A
subset of the basal cells might be epithelial stem cells for the luminal
epithelial cells. The luminal cells secrete components of prostatic fluid,
express the androgen receptor and secrete prostatespecific antigen (PSA) in an
androgen-dependent manner. The stroma is composed of fibroblasts, smooth muscle
cells, endothelial cells, dendritic cells, nerves and some infiltrating cells,
such as mast cells and lymphocytes. Some stromal cells are androgen responsive
and produce growth factors that act in a paracrine fashion on the epithelial
cells. This stromal–epithelial crosstalk is an important regulator of the
growth, development and hormonal responses of the prostate. The well-organized secretory glandular structure in the
normal prostate, accentuated here by immunostaining for E-cadherin, becomes
disrupted in invasive prostate cancer.
Prostate cancer originates most often in the basal and
luminal cells. There is an ongoing debate as to the cell of origin but we shall
not discuss that here, we have elsewhere. Yet it is also possible in rare
cases, some 2%, that the process begins with the neuroendocrine cell. These
cancers are very virulent and have a poor prognosis. Also
Neuroendocrine tumors are defined as[4]:
A tumor that forms from cells that release hormones into
the blood in response to a signal from the nervous system. Neuroendocrine
tumors may make higher-than-normal amounts of hormones, which can cause many
different symptoms. These tumors may be benign (not cancer) or malignant
(cancer). Some examples of neuroendocrine tumors are carcinoid tumors, islet
cell tumors, medullary thyroid cancer, pheochromocytomas, neuroendocrine
carcinoma of the skin (Merkel cell cancer), small cell lung cancer, and large
cell neuroendocrine carcinoma (a rare type of lung cancer).
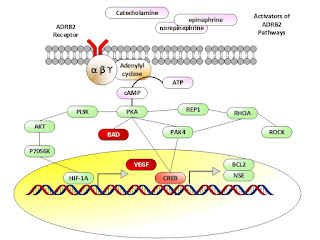
Braadland et al present the pathway activation as shown
below. They focus on the gene ADRB2. This gene is defined as follows[5]:
This gene encodes beta-2-adrenergic receptor which is a
member of the G protein-coupled receptor superfamily. This receptor is directly
associated with one of its ultimate effectors, the class C L-type calcium
channel Ca(V)1.2. This receptor-channel complex also contains a G protein, an
adenylyl cyclase, cAMP-dependent kinase, and the counterbalancing phosphatase,
PP2A. The assembly of the signaling complex provides a mechanism that ensures
specific and rapid signaling by this G protein-coupled receptor. This gene is
intronless. Different polymorphic forms, point mutations, and/or downregulation
of this gene are associated with nocturnal asthma, obesity and type 2 diabetes.
Simply noted, some one of the three activators as noted
activate the ADRB2 pathway ultimately releasing VEGF and other promoters.
Braadland et al comment on the above as follows:
Cyclic
AMP produced in response to adrenergic stimulation binds
the regulatory subunit of PKA and the activated catalytic subunit is released. The catalytic subunit may translocate to the nucleus and
phosphorylate cAMP responsive element binding protein (CREB), which induces the
expression of e.g., neuron specific enolase/enolase 2 (ENO2,
a neuroendocrine marker), and B-cell CLL/lymphoma 2 (BCL2,
encoding an anti-apoptotic protein). PKA-induced phosphorylation of
CREB may either be direct or indirect through regulation of p21-activated
protein kinase 4 (PAK4) and/or ERK activity. stress may also promote
apoptosis-resistance through PKA-dependent phosphorylation of BCL2-associated
agonist of cell death (BAD), as shown. Furthermore, PKA may inhibit
the ras homolog family member A (RhoA) – Rho-associated PKA (ROCK) pathway
leading to neurite outgrowth either directly or mediated through either Rap1, a
member of the RAS oncogene family, or PAK4. Rap1 is also possibly
involved in PKA-induced regulation of ERK activity. Finally, PKA-mediated effects of adrenergic stimuli
up-regulate vascular endothelial growth fac-tor (VEGF) levels and HUVEC
capillary tube formation via the PI3K/AKT/p70S6K/HIF-1α pathway. Besides regulating the
transcription factor activity of CREB and HIF-1α, the ADRB2/cAMP/PKA signaling pathway has been
shown to stimulate the androgen receptor responsive gene transcription
As Zahalka et al note:
Solid tumors depend on angiogenesis to sustain their
growth. The transition from hyperplasia to highly vascularized growing tumor,
referred to as the “angiogenic switch,” is a state in which proangiogenic
factors—such as vascular endothelial growth factor (VEGF) and other secreted
angiocrine factors—predominate over antiangiogenic signals. During development,
peripheral nerves associate closely with growing blood vessels, organizing
vascular pattern, a phenomenon that has also been described in models of wound
healing. Emerging studies suggest that nerves can also regulate
tumorigenesis. Sympathetic nerve fibers deliver adrenergic signals that act via
b-adrenergic receptors (bAdRs) expressed in the tumor microenvironment. However,
the cellular target(s) and molecular mechanism( s) responsible for neural
regulation of cancer are not known and may provide novel therapeutic avenues.
They summarize as follows:
1.
Adrenergic nerves regulate
angiogenesis in early tumor growth
2.
Endothelial ADRB2 controls
the angiogenic switch
3.
ADRB2 regulates oxidative
metabolism in angiogenic prostate endothelial cells
4.
Increased endothelial COA6
activity mediates the shift toward oxidative phosphorylation
The issue of neuroendocrine cells in PCa has received a
considerable amount of attention. De novo NE PCa is very aggressive and has a
very high mortality rate in less than just one year. However NE PCa is
fortunately rare. Yet NED in metastatic PCa leads to CRPC, namely androgen
blocking no longer works. In this paper we have reviewed some of the key issues
and have tried to do so by assembling the empirically provided data and then
logically creating a rational system structure amenable for a therapeutic
attack.
Beta blockers have been used for decades. Typical ones are propranolol
and timolol. As Lu et al have noted in a
meta study regarding the use of the blockers:
In summary, though there are some limitations in this
study, we observed reduced cancer-specific mortality among prostate cancer
patients taking beta-blockers. However, we did not observe any effect of
beta-blocker use on all-cause mortality in this meta-analysis. Taken together
with studies in other cancer types and in preclinical models, our findings
indicate a beneficial effect of beta-blockers on survival in patients with
prostate cancer. Therefore, beta-blockers may be considered a promising
therapeutic approach for adjuvant therapy in prostate cancer. Further clinical
trials must be explored in larger patient cohorts.
The question is: is the receptor we have focused on herein
the most effective one? Recall that the neurotransmitters we have discussed work
as follows[6]:
Thus the flow of control can be readily intercepted via a
beta blocker. There are several Beta receptors (labeled 1, 2, 3) but we should
ask if the pathways are fully defined.
As we noted above, accepting the targeting of the Beta
adrenergic receptors, we are doing so because we are led logically to
understand their role in controlling promotor proteins which in turn generate
proteins that effect growth outside of the endocrine cell. That is we have demonstrated
the pathway logic leading to the neuroendocrine paradigm initially introduced. As
Braadland et al note:
The reports on effects of β-blockers on mortality in
other cancer types brings forth an important question: are the in vivo effects
of β-blockers
mediated by common tissue specific/non-specific attributes, or are the effects
indirect (i.e., systemic or neural effects facilitated by other local or
distant tissue expressing ADRBs)? β-
blockers probably have an effect on immune responses, hormone levels,
angiogenesis, neurogenesis, and at the metastatic niche. In the prostate,
stromal cells proximal to tumor tissue express ADRBs, and may exert the effect,
which may also explain the discrepancy between cell line results and in vivo
data. It is also worth noting that the
majority of β-blockers
are targeting β1-adrenergic
receptors or both β1-
and β2-adrenergic
receptors, whereas ADRB2 has been the receptor mediating the effects on cancer
cells. Another plausible explanation lies in the antagonistic mechanism of action. Propranolol, for example, a
commonly used antagonist in vitro, has been shown to function as an inverse
agonist, and can thus lower the β-adrenergic
receptor’s activity below its’ basal level. In clinical practice, however,
numerous β-blockers
are used, and their mechanisms of action vary. Furthermore, the differences
observed could be dose-dependent, as it is difficult to measure the dose in
patient tissue, whereas this parameter can be controlled in cell lines and
animal models. We anticipate that ADRB
antagonists will reduce the development of neuroendocrine prostate cancers, but
this has not yet been addressed in any publications. More studies are needed to
unravel whether β-blockers
can play a role in future tailored prostate cancer therapy.
Thus as we asked at first, the logical basis, there seems to
be a putative reason for the efficacy of a beta blocker.
Is this the best target or are there many others which may
be used separately or in parallel? As Qi et al have previously noted:
Neuroendocrine (NE) phenotype, seen in >30% of
prostate adenocarcinomas (PCa), and NE prostate tumors are implicated in
aggressive prostate cancer. Formation of NE prostate tumors in the TRAMP mouse
model was suppressed in mice lacking the ubiquitin ligase Siah2, which
regulates HIF-1a availability. Cooperation between HIF-1a and FoxA2, a
transcription factor expressed in NE tissue, promotes recruitment of p300 to
transactivate select HIF-regulated genes, Hes6, Sox9, and Jmjd1a. These
HIF-regulated genes are highly expressed in metastatic PCa and required for
hypoxia-mediated NE phenotype, metastasis in PCa, and the formation of NE
tumors. Tissue-specific expression of FoxA2 combined with
Siah2-dependent HIF-1a availability enables a transcriptional program required
for NE prostate tumor development and NE phenotype in PCa. Our results provide
insight into regulation and function of the FoxA2/HIF-1a complex in determining
NE prostate tumor formation and NE phenotype, an important component of
metastatic prostate adenocarcinomas. These results also point to a role for
Siah2 in determining tumor differentiation. Siah2 loss has little effect on development and growth of
the prostate luminal epithelium but decreases initiation of NE carcinomas and, consequently,
the metastatic burden in the TRAMP model. We show that partial deletion of
Siah1a on a Siah2 null background fully ablated NE tumor formation, suggesting
that both Siah2 and Siah1 are required to enable the development of prostate NE
tumors. As HIF-1a is stabilized under hypoxia and FoxA2 is expressed in NE
tissues, our findings suggest conditional and spatial cooperation between these
two factors under specific tissue and oxygen requirements. Siah2-dependent regulation of HIF coupled with
NE-specific expression of FoxA2 provides a framework for a specific tumor
differentiation program associated with a highly metastatic phenotype.
Thus there is a certainty regarding the NE Type being an
aggressive indicator but the question remains is the ADRB2 receptor the primary
driver and is VEGF the primary subsequent driver. The above brief discussion
opens the door for a substantial expansion of activity. Notwithstanding this, however,
this observation does present an interesting path.
1.
Albany et al, Epigenetics
in Prostate Cancer, Prostate Cancer Volume 2011, Article ID 580318, 12 pages,
2011
2.
Anastasiadou, E., F. Slack,
Malicious exosomes, Science, 19 DECEMBER 2014 SCIENCE sciencemag.org VOL 346 ISSUE 6216, pp 1459-1460.
3.
Ayala GE, Dai H, Powell M,
et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin
Cancer Res 2008; 14: 7593-603.
4.
Beltran, et al, Molecular
characterization of neuroendocrine Prostate cancer and identification of new
Drug Targets, November 2011, Cancer Discovery, p 487
5.
Berghe W., Epigenetic
impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of
our epigenomes, Pharmacological Research 65 (2012) 565– 576
6.
Berman-Booty and Knudsen,
Models of neuroendocrine prostate cancer, Bioscientifica, 2015
7.
Braadland, et al, β-adrenergic receptor signaling
in prostate cancer, Frontiers in Oncology, Genitourinary Oncology, January 2015,
Volume 4, Article 375
8.
Braadland et al, Low
beta(2)-adrenergic receptor level may promote development of castration
resistant prostate cancer and altered steroid metabolism. OncoTarget, 7(2):
1878-1894, 2015
9.
Braicu, C., et al, Exosomes
as divine messengers: are they the Hermes of modern molecular oncology?, Cell
Death and Differentiation (2015) 22, 34–45
10. Brooks, C., W. Gu, How does SIRT1 affect metabolism, senescence
and cancer?, Nature Reviews Cancer 9, 123-128 (February 2009)
11. Chen and Ayala, Innervating Prostate Cancer, NEJM 15 Feb 2018.
12. da Silva, H., et al, Dissecting Major Signaling Pathways
throughout the Development of Prostate Cancer, Hindawi Publishing Corporation
Prostate Cancer Volume 2013, Article ID 920612, 23 pages
13. Demaitre, L., Medieval Medicine, Prager (Santa Barbara, CA) 2013
14. Di Sante, G., et al, Loss of Sirt1 Promotes Prostatic
Intraepithelial Neoplasia, Reduces Mitophagy, and Delays Park2 Translocation to
Mitochondria
15. Dominy, J., et al, The Deacetylase Sirt6 Activates the
Acetyltransferase GCN5 and Suppresses Hepatic Gluconeogenesis, Molecular Cell
48, 900–913, December 28, 2012
16. Ellis and Loda, Advanced neuroendocrine prostate tumors regress
to stemness, PNAS, November 24, 2015, vol. 112, no. 4
17. Feldman and Feldman, The development of androgen-independent
prostate cancer, Nature Reviews , Cancer Volume 1 , October 2001 , 35
18. Goldstein, A., et al, Identification of a Cell of Origin for Human
Prostate Cancer, Science, Vol 329, July 2010, p 568.
19. Grigore et al, Prostate cancer and neuroendocrine
differentiation: more neuronal, less endocrine?, Frontiers in Oncology, March
2015, Volume 5, Article 37
20. Guatente, L., Sirtuins, Aging, and Medicine, NEJM, Vol 364 June
2011
21. Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor
promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer
Cell 2017; 31: 21-34.
22. Hayakawa Y, Wang TC. Nerves switch on angiogenic metabolism.
Science 2017; 358: 305-6.
23. Heinlein and Chang, Androgen Receptor in Prostate Cancer, Endocrine
Reviews 25(2):276–308, 2004
24. Hines, Z., et al, The cAMP/PKA Pathway Rapidly Activates SIRT1
to Promote Fatty Acid Oxidation Independently of Changes in NAD+, Molecular Cell
44, 851–863, December 23, 2011
25. Hu et al, Neuroendocrine differentiation in prostate cancer: a
mechanism of radioresistance and treatment failure, Frontiers in Oncology,
Genitourinary Oncology April 2015, Volume 5, Article 90
26. Labbe, D., et al, Role of Diet in Prostate Cancer, the
Epigenetic Link, Oncogene, 2014.
27. Lam, E., et al, FOXO Transcription Factors, Biochem Soc Trans
2006, pp 722-726.
28. Lefkowitz, G., et al, Follow Up Interval Prostate Biopsy of High
Grade Prostatic Intraepithelial Neoplasia is Associated with High Likelihood of
Prostate Cancer Independent of Change in Prostate Specific Antigen Levels,
Journal of Urology, Vol 168, Oct 2002.
29. Li et al, Molecular aspects of prostate cancer with
neuroendocrine differentiation, Chin J Cancer Res. 2016 Feb; 28(1): 122–129
30. Lu et al, Impact of beta-blockers on prostate cancer mortality:
a meta-analysis of 16,825 patients, OncoTargets and Therapy 2015:8 985–990
31. Magnon C, Hall SJ, Lin J,
et al. Autonomic nerve development contributes to prostate cancer progression.
Science 2013; 341: 1236361.
32. Mattern, S., Galen and the Rhetoric of Healing, Hopkins
(Baltimore) 2008.
33. Mattern, S., The Prince of Medicine, Oxford (New York) 2013
34. McGarty, T., Prostate Cancer Genomics, DRAFT 2013.
www.telmarc.com
35. Melo et al, Cancer Exosomes Perform Cell-Independent MicroRNA
Biogenesis and Promote Tumorigenesis, Cancer Cell, Volume 26, Issue 5,
p707–721, 10 November 2014.
36. Mydlo and Godec, Prostate Cancer, 2nd Ed, Academic
(Waltham MA) 2016.
37. Ott, R., et al, JunB is a gatekeeper for B-lymphoid leukemia,
Oncogene (2007) 26, 4863–4871
38. Parimi et al, Neuroendocrine differentiation of prostate cancer:
a review, Am J Clin Exp Urol 2014;2(4):273-285
39. Pastell, R., M. Nevalainen, Prostate Cancer, Humana (Totowa)
2008.
40. Pekarik, V., et al, Prostate Cancer, miRNAs, Metallothioneins
and Resistance to Cytostatic Drugs,
Current Medicinal Chemistry, 2013, 20, 534-544
41. Powell, M., et al, Disruption of a Sirt1 -Dependent Autophagy
Checkpoint in the Prostate Results in Prostatic Intraepithelial Neoplasia
Lesion Formation, Cancer Res Published OnlineFirst December 28, 2010.
42. Pulla, V., et al, IRT1 Pathway in T2DM, https://www.researchgate.net/publication/259223485_SIRT1_Pathway_in_T2DM
43. Qi et al, Siah2-Dependent Concerted Activity of HIF and FoxA2
Regulates Formation of Neuroendocrine Phenotype and Neuroendocrine Prostate
Tumors, Cancer Cell 18, 23–38, July 13, 2010
44. Renz BW, Takahashi R, Tanaka T, et al. β2
adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer
Cell 2018; 33(1): 75-90.e7.
45. Roy, S., et al, Inhibition of PI3K/AKT and MAPK/ERK pathways
causes Activation of FOXO Transcription Factor, Jrl Mol Sig 2010, pp 1-13.
46. Ruan et al, SIRT1 contributes to neuroendocrine differentiation
of prostate cancer, Oncotarget, 2018, Vol. 9, (No. 2), pp: 2002-2016
47. Shackelford, D., R. Shaw, The LKB1–AMPK pathway: metabolism and
growth control in tumour suppression, Nature Reviews, Cancer Volume 9, August
2009, 563
48. Slaby, O., et al, MicroRNAs in colorectal cancer: translation of
molecular biology into clinical application. Mol Cancer. 2009 Nov 14;8:102.
doi: 10.1186/1476-4598-8-102.
49. Sun et al, Neuroendocrine differentiation in prostate cancer, Am
J Transl Res 2009;1(2):148-162
50. Thomsen, M., et al, Loss of JUNB/AP-1 promotes invasive prostate
cancer, Cell Death & Differentiation , (19 December 2014) doi:10.1038/cdd.2014.213.
51. van der Heide, L., et al, The Ins and Outs of FOXO Shuttling,
Biochem Jrl 2004, pp 297-309.
52. Wade and Kyprianou, Profiling Prostate Cancer Therapeutic
Resistance, Int Jrl Mol Sci, 2018 V 19, No 904
53. Zahalka AH, Arnal-Estapé A, Maryanovich M, et al. Adrenergic
nerves activate an angio-metabolic switch in prostate cancer. Science 2017;
358: 321-6.
54. Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses
gastric tumorigenesis. Sci Transl Med 2014; 6: 250ra115.
[1]
See Mattern (2013) p 37-39 where there is a reasonable discussion of Galen and
his approaches. Also one could examine the interactions between Marsilius of
Padua, a Physician and Political Scientist in the 14th century with
William of Ockham, the Philosopher. Both built an understanding of the blend of
rationalism and empiricism.
[3]
See Mydlo and Godec, pp 149-153.
[6]
See Clark et al, Pharmacology, 5th Ed, Lippincott, 2012, p 43