The recent explosion in immunotherapy targeting such
elements as PD-1 or PD-L! and others has demonstrated a significant positive
step forward. However recent results indicate that there may also be a dark
side to this process.
As Sarkizova and Hacohen have noted:
The T cells of the immune system have a key role in the
identification and elimination of cells that pose a threat to the body, such as
infected cells and cancer cells. ….(authors) propose a framework to assess how
effectively tumours can be detected by T cells — a tumour property known as
immunogenicity. The authors demonstrate that their models for assigning
tumour-immunogenicity scores can be used to predict clinical responses to a
type of cancer immunotherapy called checkpoint blockade.
Most cells in the body present peptide fragments known as
antigens on their cell surface, which are generated from intracellular
proteins. Each peptide is bound in a complex with a specialized receptor called
an MHC class I protein (HLA class I in humans). T cells known as cytotoxic T
cells police the body in search of cells displaying specific antigens,
especially antigens from infectious organisms, or in the case of cancer,
antigens known as neoantigens that have arisen as a result of a mutation. If
the T-cell receptor (TCR) of a cytotoxic T cell recognizes and binds an antigen
that is not normally present, the T cell will often unleash an attack that
kills the cell displaying that antigen. TCRs are highly variable and have
slightly different antigen-binding regions, enabling the immune system to
recognize millions of antigens. Antigen binding to MHC proteins and TCR
recognition of antigen–MHC complexes are key determinants of an immune
response.
They continue with the discussion of checkpoints as follows:
Tumour cells often fight back against this immune-system
surveillance by hijacking the natural mechanisms that dampen immune responses,
which are normally intended to block autoimmmune attacks against healthy
tissue. Checkpoint-blockade therapies can block these immuno-inhibitory
signals, such as those generated by the ‘checkpoint’ PD-L1 protein4. However,
only a subset of tumours treated with such therapies regress. Therefore,
approaches are needed to identify the tumours that are most likely to respond
to immunotherapy.
Current ways of predicting the effectiveness of
checkpoint-blockade therapy rely on measuring the level of PD-L1 protein
expressed by tumour cells, counting the number of T cells in a tumour, and
estimating the number of different neoantigens that a tumour contains5. The
work by Łuksza and Balachandran and their respective colleagues offers a new
type of integrated model to predict whether a tumour will be attacked by T
cells, a characteristic that they refer to as tumour fitness (low fitness being
associated with a strong immune response against the tumour).
Thus in the best of worlds we see the result below. As Ludin
and Zon note:
PD-1 is expressed on the surface of immune cells called T
cells. When PD-1 is bound by a ligand produced by tumour cells, PD-1 signalling
renders the T cell inactive, preventing immune responses that would destroy the
tumour. Treatment with an antibody to PD-1 blocks ligand binding and so PD-1
signalling, instead promoting the PI3K signalling pathway, which is involved in
T-cell activation. As such, anti-PD-1 treatment triggers an immune response,
Wartewig et al. have demonstrated that PD-1 signalling in
a mouse model o f T cell non- Hodgkin’s lymphoma prevents proliferation of
cancerous T cells (the source of the PD-1 ligand was not defined). In these
mice, anti-PD-1 treatment can aggravate disease by reactivating the cancerous
cells to enable then continuous proliferation.
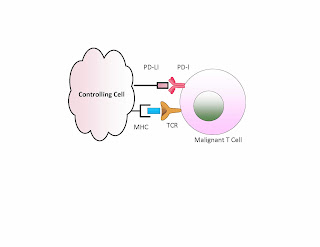
Namely a tumor cell having a PD-L1 can be attacked by the T
cells when we block this suppressor. We demonstrate this below. If PD-1 is
matched with a PD-L1 then the T cell remains inactive. If we have an antibody
used to block either PD-1 or PD-L1 then we can get T cell activation.
In contrast if the bad cell is the T cell, or if there is
such a cell present in addition to the other tumor, then removing this block
may activate the malignant T cell and off we go with a second malignancy. The example
we show below.
As Wartewig et al note:
By contrast, a homo- or heterozygous deletion of PD-1
allows unrestricted T cell growth after an oncogenic insult and leads to the
rapid development of highly aggressive lymphomas in vivo that are readily transplantable
to recipients. Thus, the inhibitory PD-1 receptor is a potent haploinsufficient
tumour suppressor in T cell lymphomas that is frequently altered in human
disease. These findings extend the known physiological functions of PD-1 beyond
the prevention of immunopathology after antigen-induced T cell activation, and have
implications for T cell lymphoma therapies and for current strategies that
target PD-1 in the broader context of immuno-oncology
Namely we have the potential that certain cells have managed
to block malignant T cells from multiplying and thus keep a hematological
cancer under control. When attacking another cancer with a blockade it may then
allow this blocked cell to proliferate.
As Giladi and Amit note:
The cells of the immune system, which patrol the blood
and dwell in tissues, have many functions. They protect the body from pathogens
and cancer, and orchestrate metabolism and the formation of organs. They are involved
in almost every activity that regulates the body’s internal environment, from the
development and remodelling of tissues to the clearance of dying cells and
debris. So their dysfunction can cause many problems
This may be an obvious statement but it sets the path for
what is to follow. The more one learns about the immune system the more we find
a complexity of cells, not just T or B cells but T cells which perform
different sets of functions. To understand this we need tools that allow us to
ascertain what happens on a single cell basis. The authors continue:
First, it is clear that many of the current categories of
immune cells, such as T cells or monocytes, encompass heterogeneous populations.
To probe cellular complexity, researchers must therefore cast their nets wide,
and try to collect all immune cells within a tissue or region of interest. This
is a very different approach from that used with methods based on cell-surface
markers, which aim to obtain as pure a sample as possible.
Second, success will depend, in part, on the extent to
which researchers preserve the states of cells and the original composition of
a tissue. Cell stress or death should be minimized to ensure that tissue
preparation does not favour specific cell types. …
Third, bioinformaticians will need to develop scalable
and robust algorithms to cope with greater numbers of cells, conflicting or
overlapping programs of gene expression and fleeting developmental stages.
Fourth, after researchers have characterized all of the
immune cells in a sample, they will need to find molecular markers that can be
used to either enrich or deplete certain cell types in further samples.
The issue then is that it is essential to have in the
"tool box" methods to carefully examine individual cells in situ.
There are clear indications that cell interactions are complex, and also are
extremely dynamic. The real question is: what level of depth of differentiation
is essential for what level of patient care? As we noted above, there are cases
where PD-1 blockage can on the one hand activate the immune system against the
cancer and on the other hand suddenly activate dormant malignancies. Is this
then just a "whack a mole" strategy against cancer, namely attacking
one only to have then to attack a second resulting from the success of the
first?
Fox and Loeb had previously attacked this issue from the
perspective of breast cancer. They noted:
The total number of mutations that a tumour genome
carries, including those present in only a small subset of cells, may in fact underlie
the aggressiveness of different cancer subtypes. For example, the extent of
genetic diversity within a tumour, and its divergence from normal tissue,
probably influences the ability of the immune system to distinguish malignant
cells from normal cells. Identifying the mechanisms by which cancer cells
generate mutational heterogeneity may therefore present previously unexplored
targets.
Single-cell sequencing will allow us to detect rare
mutant subpopulations hidden within cancers that could expand and lead to drug
resistance, and thus to avoid unnecessary and potentially harmful
administration of ineffective, toxic therapies. Ultimately, the exceptional
plasticity of the tumour genome may well prove to be a key characteristic of
cancer11 and a major, as yet untapped, therapeutic vulnerability.
There clearly is a growing need to perform a multiplicity of
single cell analyses. However this is a complex spatio-temporal result, with a
great deal of extraneous information. We should understand how cells genetic
makeup changes as a function of time and of location. Location is itself complex
because it refers to what cells are adjacent and even just close. Furthermore
many gene expressions or suppressions are irrelevant, just chaff in an attempt
to track a target. The desire to measure single cells is but a first step. A
"model" or paradigm of what is essential for understanding the
"system" is essential.
References:
1. Fox and Loeb, One cell at a time, Nature, August 2014.
2. Giladi and Amit, Immunology, one cell at a time, 6 July 2017 |
Vol 547 | Nature | 2 7
3. Ludin and Zon, The dark side of PD-1 receptor inhibition,
Nature, 7 December 2017, VOL 552, p 41
4. Lukasz et al, A neoantigen fitness model predicts tumour response
to checkpoint blockade immunotherapy , Nature November 2017
5. Sarkizova and Hacohen, How T cells spot tumour cells, Nature
November 23 2017.
6. Wartewig et al, PD-1 is a haploinsufficient suppressor of T cell
lymphomagenesis, 7 December 2017, Vol 552, Nature, p. 121