As Weinberg notes, there is the theory of clonal development of cancer which states that the cancer cells are pluripotent and have developed from a single source and that they have the capability of reproducing and do so in an autonomous manner (see Weinberg, Cancer, pp 416-417). Then there is the theory of the cancer stem cell, the theory which states that there is the equivalent of a stem cell as we know in blood cells, which have the capability but that the majority of malignant cells do not necessarily have that capacity.
In an NIH report they define cancer stem cells as follows:
A consensus panel convened by the American Association of Cancer Research has defined a CSC as "a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor." It should be noted that this definition does not indicate the source of these cells—these tumor-forming cells could hypothetically originate from stem, progenitor, or differentiated cells.
As such, the terms "tumor-initiating cell" or "cancer-initiating cell" are sometimes used instead of "cancer stem cell" to avoid confusion. Tumors originate from the transformation of normal cells through the accumulation of genetic modifications, but it has not been established unequivocally that stem cells are the origin of all CSCs.
The CSC hypothesis therefore does not imply that cancer is always caused by stem cells or that the potential application of stem cells to treat conditions such as heart disease or diabetes, as discussed in other chapters of this report, will result in tumor formation. Rather, tumor-initiating cells possess stem-like characteristics to a degree sufficient to warrant the comparison with stem cells; the observed experimental and clinical behaviors of metastatic cancer cells are highly reminiscent of the classical properties of stem cells.
The stem cell theory, and there seems now to be significant evidence of its validity in prostate cancer, is principally that the clonal theory has merit to a point but that the development is more complex and the cancer stem cell plays a critical role in fostering growth of the cancer cells, most of which has less aggressive a growth characteristic if any at all.
Lawson and Witte present a recent overview of this concept as applied to the prostate and PCa. Recent studies apparently indicate that the cancer stem cells, CSC, are necessary to sustain later stages of the development of the malignancy. Only a small subpopulation of the cancer cells, the CSC population, has a demonstrated ability to maintain the malignancy as well.
Lawson and Witte present two theories of this CSC process. One is called the stochastic theory which is that all cells are equally malignant. The other theory, the one for CSC, called the hierarchical theory is that only the CSC has the ability to multiply. These two are graphically depicted below. The CSC or in this case the PSC, prostate stem cell, yields a TAC, or transition amplifying cells, then yield progenitor cells, LP or BP, and then finally a luminal or basal cell. This is slight contrast to the Goldstein model. This model applies for both benign as well as cancer cells, at least as viewed by Lawson and Witte.
Lawson and Witte present two theories of this CSC process. One is called the stochastic theory which is that all cells are equally malignant. The other theory, the one for CSC, called the hierarchical theory is that only the CSC has the ability to multiply. These two are graphically depicted below. The CSC or in this case the PSC, prostate stem cell, yields a TAC, or transition amplifying cells, then yield progenitor cells, LP or BP, and then finally a luminal or basal cell. This is slight contrast to the Goldstein model. This model applies for both benign as well as cancer cells, at least as viewed by Lawson and Witte.

Now if one looks at the CSC theory, then we see a CSC has progeny, and yet those progeny may not have the ability to multiply. Thus the explosive exponential growth of cancer is not as clear in a CSC model, because almost all of the progeny of the CSC are no reproducing progeny. Thus the growth models for a CSC based malignancy are more complex and are dependent on limited CSC reproduction and non-CSC reproduction.
However the CSC model also argues for there being some CSC support for the progeny which are not CSC. The dynamics of cell growth then becomes quite complex here, for the stem cells replicate themselves at a slow rate but are replicating other cells at a higher rate. However the other cells do not replicate themselves they just go through a standard cell process. If the cells are benign then they go through apoptosis as seen in red blood cells and the skin keratinocytes.
As the Guardian states:
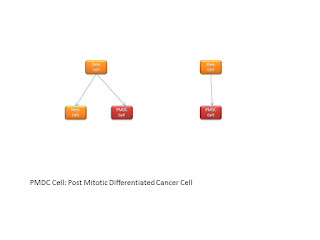
Now we can view the stem cells as shown below. There is a stem cell which can give rise to a new stem cell of ultimately a Post Mitotic Differentiated Cancer Cell. The PMDC cannot replicate, whereas the stem cell can. For metastasis it is thus necessary to send out a few stem cells, not PMDC cells.
Then there is a cascade of these cells into a Transit Amplifying Cell and then finally the Post Mitotic Differentiated Cancer Cell.
Then we can see the stem cells replicating as follows:
However the CSC model also argues for there being some CSC support for the progeny which are not CSC. The dynamics of cell growth then becomes quite complex here, for the stem cells replicate themselves at a slow rate but are replicating other cells at a higher rate. However the other cells do not replicate themselves they just go through a standard cell process. If the cells are benign then they go through apoptosis as seen in red blood cells and the skin keratinocytes.
As the Guardian states:
An emerging, although highly controversial, answer to this question is that cancer's immortality, too, is borrowed from normal physiology. The human embryo and many of our adult organs possess a tiny population of stem cells that are capable of immortal regeneration. Stem cells are the body's reservoir of renewal. The entirety of human blood, for instance, can arise from a single, highly potent blood-forming stem cell (called a haematopoietic stem cell), which typically lives buried inside the bone marrow. Under normal conditions, only a fraction of these blood-forming stem cells are active; the rest are deeply quiescent – asleep. But if blood is suddenly depleted, by injury or chemotherapy, say, then the stem cells awaken and begin to divide with awe-inspiring fecundity, generating cells that generate thousands upon thousands of blood cells. In weeks, a single haematopoietic stem cell can replenish the entire human organism with new blood - and then, through yet unknown mechanisms, lull itself back to sleep.
Something akin to this process, a few researchers believe, is constantly occurring in cancer – or at least in leukaemia. In the mid-1990s, John Dick, a Canadian biologist working in Toronto, postulated that a small population of cells in human leukaemias also possess this infinite self-renewing behaviour. These "cancer stem cells" act as the persistent reservoir of cancer – generating and regenerating cancer infinitely. When chemotherapy kills the bulk of cancer cells, a small remnant population of these stem cells, thought to be intrinsically more resistant to death, regenerate and renew the cancer, thus precipitating the common relapses of cancer after chemotherapy. Indeed, cancer stem cells have acquired the behaviour of normal stem cells by activating the same genes and pathways that make normal stem cells immortal – except, unlike normal stem cells, they cannot be lulled back into physiological sleep. Cancer, then, is quite literally trying to emulate a regenerating organ – or perhaps, more disturbingly, the regenerating organism. Its quest for immortality mirrors our own.
Now we can view the stem cells as shown below. There is a stem cell which can give rise to a new stem cell of ultimately a Post Mitotic Differentiated Cancer Cell. The PMDC cannot replicate, whereas the stem cell can. For metastasis it is thus necessary to send out a few stem cells, not PMDC cells.
Then there is a cascade of these cells into a Transit Amplifying Cell and then finally the Post Mitotic Differentiated Cancer Cell.
Then we can see the stem cells replicating as follows:
To better understand we quote Lawson and Witte as follows:
Models of prostate epithelial differentiation. The traditional model for prostate epithelial differentiation proposes that PSCs residing in the basal cell layer give rise to intermediate, transit-amplifying cells that produce large numbers of terminally differentiated secretory luminal cells …. This model implies a linear differentiation scheme in which basal and luminal cells comprise one lineage and basal cells are essentially luminal cell progenitors …
This hypothesis is supported by the existence of cells of intermediate phenotype that express both basal- and luminal cell–specific cytokeratins in both fetal and adult stages of prostate development … Intermediate cells can also be identified in in vitro cultures of primary prostate epithelium … Several studies have also suggested basal cells can differentiate into luminal cells in vitro … Alternative theories for prostate epithelial differentiation propose basal and luminal cells may represent separate epithelial lineages … This is similar to prevailing models for epithelial differentiation in the mammary gland, a tissue that is anatomically and functionally analogous to the prostate …
Now there have been several others who have examined the stem cell model for PCa. Another of recent merit is that of Hurt et al. They summarize their work as follows:
Recent evidence supports the hypothesis that cancer stem cells are responsible for tumor initiation and formation. Using flow cytometry, we isolated a population of CD44+CD24- prostate cells that display stem cell characteristics as well as gene expression patterns that predict overall survival in prostate cancer patients. CD44+CD24- cells form colonies in soft agar and form tumours in NOD/SCID mice when as few as 100 cells are injected.
Furthermore, CD44+CD24- cells express genes known to be important in stem cell maintenance, such as BMI-1 and Oct-3/4. Moreover, we can maintain CD44+CD24- prostate stem-like cells as non-adherent spheres in serum-replacement media without substantially shifting gene expression. Addition of serum results in adherence to plastic and shifts gene expression patterns to resemble the differentiated parental cells.
Thus, we propose that CD44+CD24- prostate cells are stem-like cells responsible for tumor initiation and we provide a genomic definition of these cells and the differentiated cells they give rise to. Furthermore, gene expression patterns of CD44+CD24- cells have a genomic signature that is predictive of poor patient prognosis. Therefore, CD44+CD24- LNCaP prostate cells offer an attractive model system to both explore the biology important to the maintenance and differentiation of prostate cancer stem cells as well as to develop the therapeutics, as the gene expression pattern in these cells is consistent with poor survival in prostate cancer patients.
Jordan et al characterize cancer stem cells as having three characteristics:
1. Self-Renewal: at the end of mitosis of the stem cell, either one or both retain all the characteristics of the parent. The stem cell goes through a mitotic doubling and when it does it always retains one or two stem cell daughters.
2. Capability to generate multiple lineages. This means that a stem cell can generate offspring which can become anyone of many cell types.
3. Potential to proliferate extensively. The cell can keep replicating, it has no limitation within reason and thus contains the elements ultimately for metastasis.
A normal stem cell may mutate to a cancer stem cell or a normal progenitor cell may morph back to a cancer stem cell.
As Delarbra et al state:
Although monoclonal in origin, most tumors appear to contain a heterogeneous population of cancer cells. This observation is traditionally explained by postulating variations in tumor microenvironment and coexistence of multiple genetic subclones, created by progressive and divergent accumulation of independent somatic mutations.
An additional explanation, however, envisages human tumors not as mere monoclonal expansions of transformed cells, but rather as complex tridimensional tissues where cancer cells become functionally heterogeneous as a result of differentiation.
According to this second scenario, tumors act as caricatures of their corresponding normal tissues and are sustained in their growth by a pathological counterpart of normal adult stem cells, cancer stem cells.
The statement starts with the accepted monoclonal hypothesis and then departs to a polyclonal alternative view. It retains the CSC, cancer stem cell, paradigm for solid tumors as well. In the context of HGPIN we see a change in the cells and we have heard the argument that they have made one or several of the unchangeable steps towards PCa.
Thus using the CSC theory one would expect that it would be from one or several of these cells that PCa would arise. In addition, we could assume that there is no unique pathway mutations or changes which result in PCa but a plethora of them. Simply stated, cancer is complex, it finds ways to migrate forward no matter what the path.
A recent study has focused on the stem cell and its dynamics. They state:
This should be an interesting area to follow.
References:
A recent study has focused on the stem cell and its dynamics. They state:
The method, published in the online journal PLoS ONE in January, may rev up efforts to develop stem cell therapies for Alzheimer's, Parkinson's and other diseases. It may also help get to the root of the cancer-stem cell theory, which puts forth the idea that a tiny percentage of loner cancer cells gives rise to tumors.
"Math is going to be the new microscope of the 21st century because it is going to allow us to see things in biology that we cannot see any other way," said Brent Reynolds, Ph.D., an associate professor of neurosurgery at UF's McKnight Brain Institute and a member of the UF Shands Cancer Center. "Stem cells and the cells that drive cancer may be as infrequent as one in 10,000 or one in 100,000 cells. The problem is how do you understand the biology of something whose frequency is so low?"
Inspired by a 2004 essay by Joel E. Cohen, Ph.D., of The Rockefeller University and Columbia University that described the explosive synergy between mathematics and biology, Reynolds and postdoctoral associate Loic P. Deleyrolle set out to build an algorithm that could determine the rate stem cells and cancer stem cells divide.
High hopes to treat or prevent diseases have been pinned on these indistinguishable cells, which are often adrift in populations of millions of other cells. Scientists know stem cells exist mainly because their handiwork is everywhere — tissues heal and regenerate because of stem cells, and somehow cancer may reappear years after it was thought to be completely eliminated.
This should be an interesting area to follow.
References:
Dalerba, P., et al, Cancer Stem Cells: Models and Concepts, Stem Cells, 2008 pp 267-284.
Hurt, E., et al, CD44+CD44- Prostate Cells are Early Cancer Progenitor Stem Cells that Provide a Model for Patients with Poor Prognosis, Brit Jrl Can 2008 pp 756-765.
Jordan, C., et al, Cancer Stem Cells, NEJM 2006,
Lawson, D., O. Witte, Stem Cells in Prostate Cancer Initiation and Progression, Jrl Clin Inv, 2007 pp 2044-2050.